Development history of ALZ-801/valiltramiprosate
In 2013, Alzheon licensed ALZ-801, preclinical and clinical data for ALZ-801 predecessor molecule tramiprosate from Bellus Health. Tramiprosate was evaluated in more than 2,000 patients with Alzheimer’s in two Phase 3 clinical trials by Bellus Health Inc.: a North American trial and a European trial. The objectives of these trials were to evaluate the efficacy and safety of tramiprosate in patients with Mild to Moderate Alzheimer’s disease over a 78-week treatment period. Most commonly observed adverse events in these trials were nausea, vomiting and weight loss.
Blood levels of tramiprosate in these patients showed high variability and the analysis of all subjects in the North American study did not show a statistically significant difference between the treatment and placebo groups for the primary efficacy endpoints. Subsequently, the European trial was terminated early after the results of the North American study became known.
Alzheon completed post hoc analyses of Bellus’ Phase 3 North American trial results based on APOE4 status, a pre-defined variable in Bellus’ statistical plan. A systematic analysis of the dataset revealed a promising efficacy signal in a subgroup of patients who were APOE4 carriers. This signal was strongest in mild AD patients who carried 2 copies of the APOE4 allele (APOE4/4). These findings guided development of ALZ-801, a valine prodrug of tramiprosate, which is initially focused on APOE4 carriers.
ALZ-801 showed improved gastrointestinal tolerability and reliable absorption into the bloodstream in Phase 1 and Phase 2 clinical trials. In completed 2-year Phase 2 biomarker trial, ALZ-801 treatment lowered plasma p-tau181, reduced the rate of hippocampal atrophy, and stabilized clinical AD progression in APOE4 carriers with Early AD and high levels of amyloid and tau pathology.
Impact of ALZ-801 treatment in APOE4 carriers
- ALZ-801 treatment stabilized clinical progression in APOE4 Early AD carriers with high levels of amyloid & tau pathology
- ALZ-801 observed response in line with prior tramiprosate data
- Reduction in rate of hippocampal atrophy compared to ADNI consistent with AD disease modification modification
- Clinical benefits robustly correlated with brain volume preservation
- Biomarker data strongly point towards improved clearance of Aβ from brain to plasma
- Supports anti-oligomer action of ALZ-801 that maintains Aβ in soluble monomeric form
- Durable statistically significant plasma p-tau181 lowering supports downstream effects on AD pathology
- Phase 2 safety data continued to show favorable safety profile of ALZ-801 with no organ toxicity or ARIA-E events in APOE4 carrier population
ALZ-801/valiltramiprosate development history
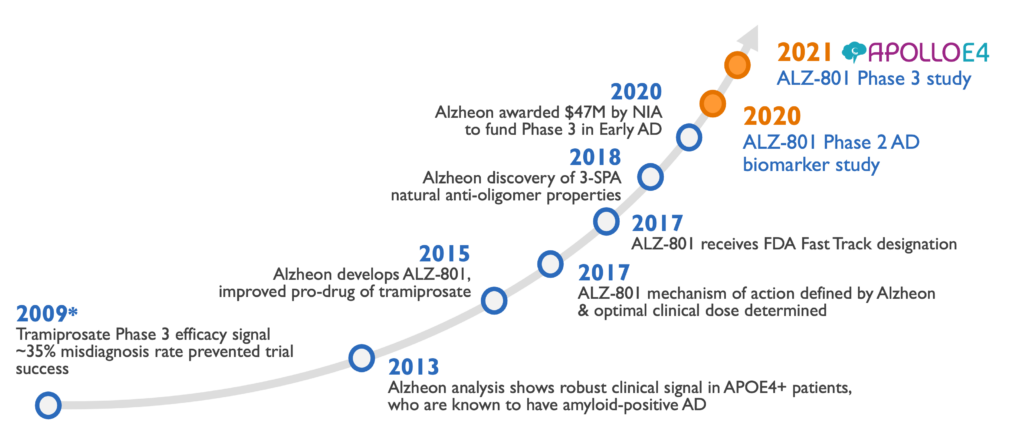
*Active metabolite of ALZ-801, licensed by Alzheon from Bellus Health/Neurochem in 2013
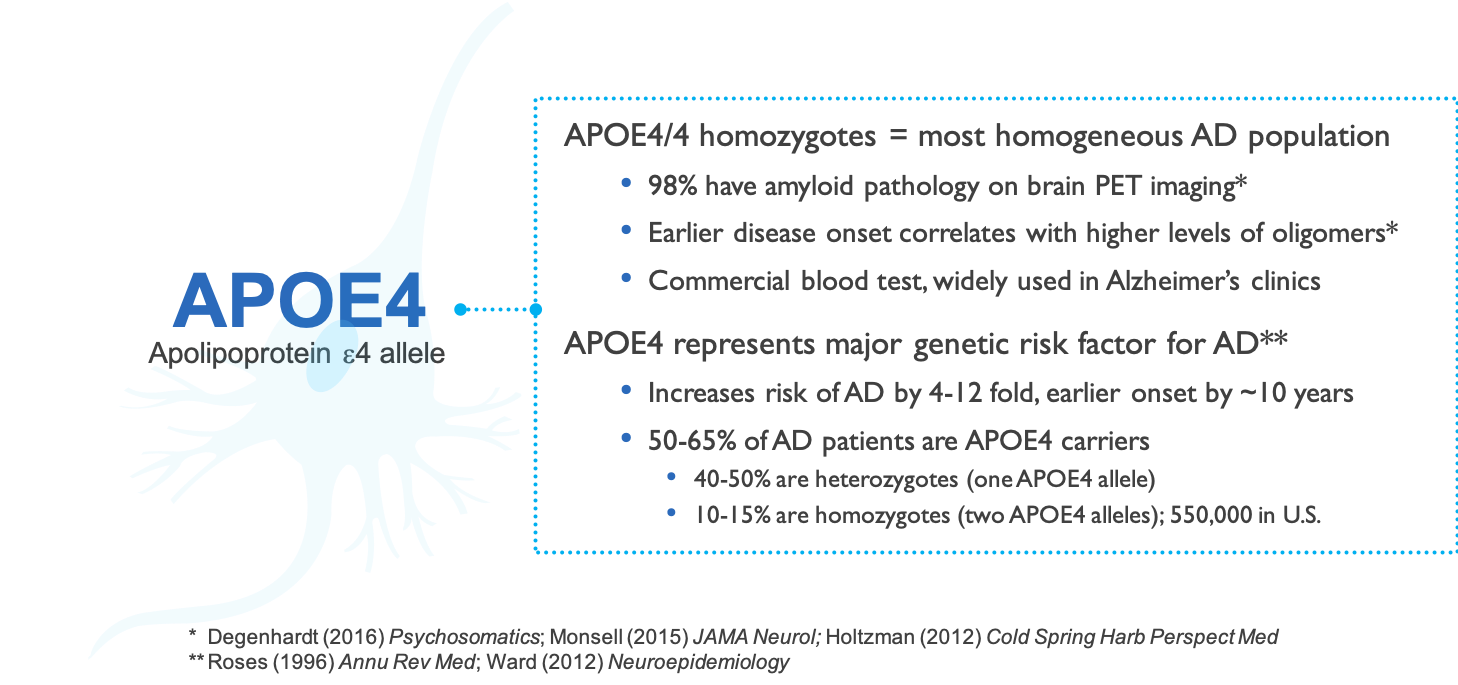
APOE4/4 patients at high risk of disease progression
Cognition stabilized in tramiprosate Phase 3
Subgroup: APOE4/4 homozygotes with Mild AD, MMSE 22-26^
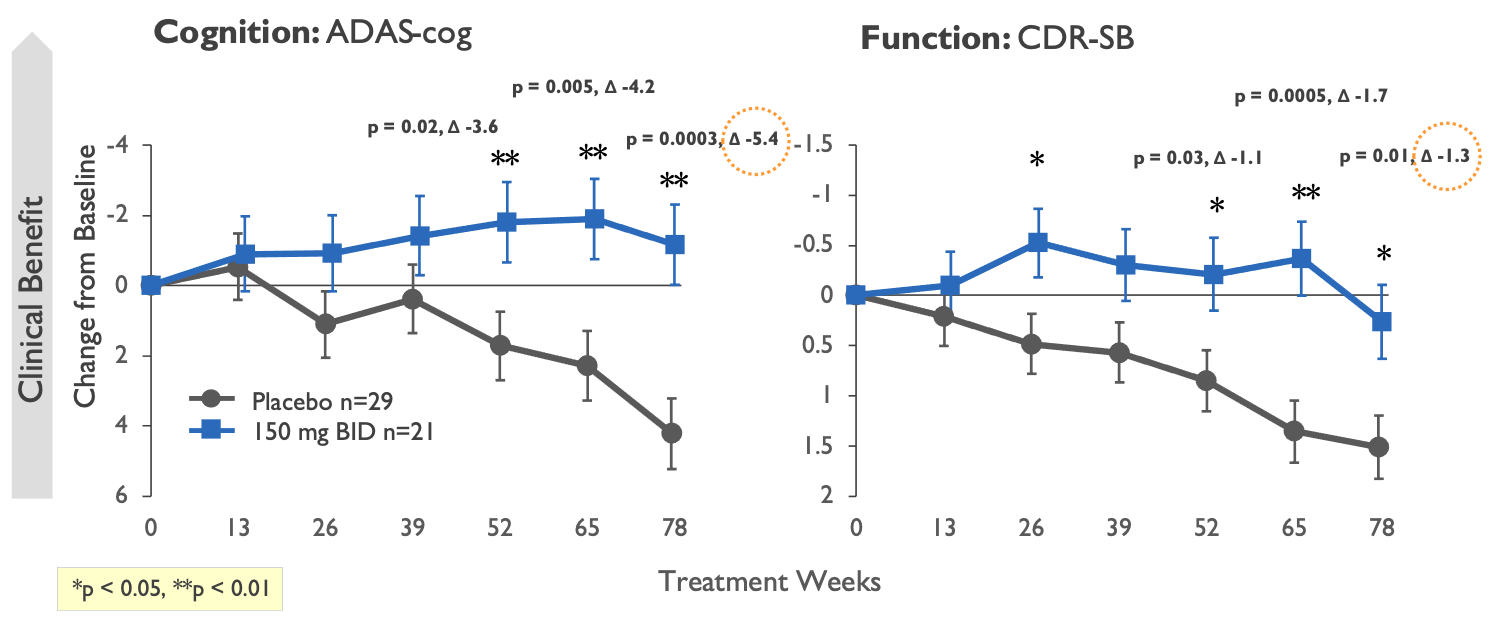
^Post hoc analysis; Alzheon publication: Abushakra (2017) J Prev Alz Dis
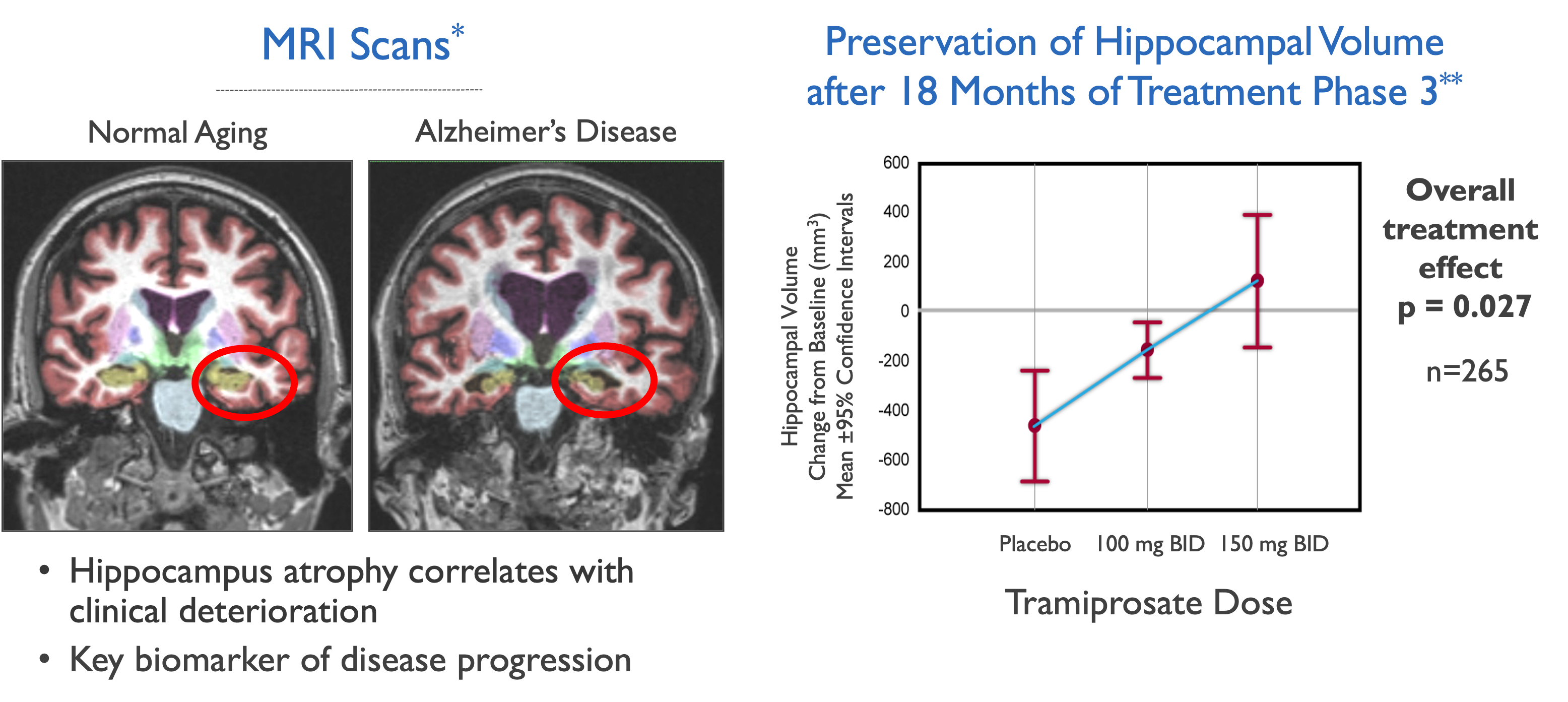
*Images courtesy of Dr. Jeff Cummings – Cleveland Clinic
**Gauthier (2009) Nutr Health Aging
Preservation of hippocampal volume over 18 months
Completed tramiprosate Phase 3 clinical summary
Confirmed APOE4/4 targeting, optimal dose & robust clinical effect
- Robust efficacy in cognition & daily function in APOE4 subgroups
- Disease stabilized over 18 months in APOE4/4 Mild AD subjects
- No hippocampal atrophy over 18 months
- Clinical benefits persist over 2.5 years of treatment
- Favorable safety
- Well tolerated, mild nausea & vomiting
- No brain edema (MRI subgroup n=426)
- Long-term safety up to 1.5 years (n=1,600) & 2.5 years (n=400)