Alzheon Pipeline
Our lead investigational product, ALZ-801 (valiltramiprosate), is in Phase 3 for Alzheimer’s
Focused on early Alzheimer’s disease
Targeted patient selection based on APOE4 genotype
Granted Fast Track Designation by FDA for development in Alzheimer’s disease
ALZ-801 is an improved prodrug of tramiprosate
Promising clinical signals in a subset of Alzheimer’s patients
Well tolerated, long-term safety evaluated in 2,000 patients at effective clinical dose
Our next generation candidate is ALZ-1903
New chemical entity
More potent inhibitor of amyloid misfolding
Discovery Platform
Rich and diverse compound libraries enabled by the novel mechanism of action
Designed to inhibit the misfolding of proteins associated with neurodegenerative diseases
ALZ-801 development starts with patients most likely to benefit from our compound
First indication: 2.3 million patients
– Early AD in APOE4/4 homozygotes
Expansion indications: 10.8 million patients
– Early AD in APOE4 carriers (5.3 M)
– Prevention of AD in APOE4/4 homozygotes (5.5 M)
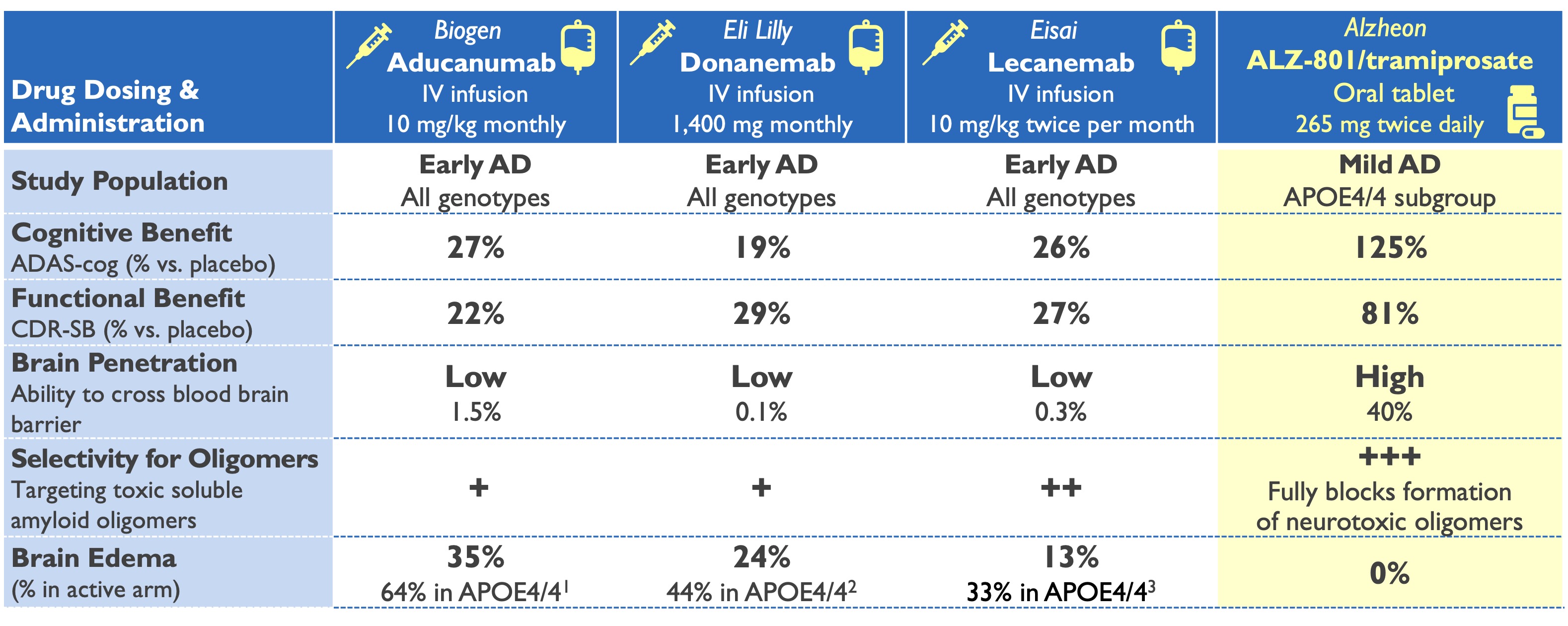
- Well-tolerated oral tablet ideally suited to needs of Alzheimer’s families
- No burdensome IV infusion or MRI monitoring for brain edema
- Strong value proposition of 18-month delay in disease progression
Source: DRG 2020 Report, assumes 98% diagnostic rate in APOE4/4 and 70% diagnostic rate in APOE3/4 in 2024
Misfolded proteins cause neurodegenerative disorders
Neurotoxic proteins behave like prions, build-up & damage brain cells
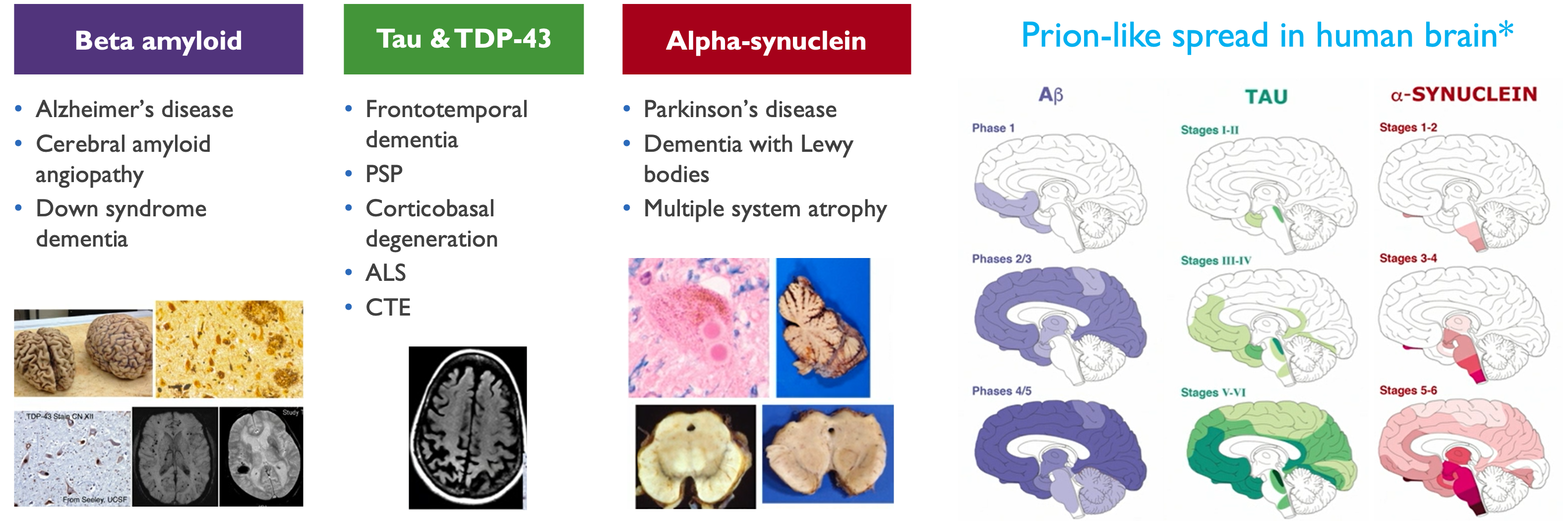
*Goedert (2017) Brain
Scientific insights pioneered by Alzheon & applied to development program
- Effective inhibition of brain toxins causing Alzheimer’s – addressed by ALZ-8011
- ALZ-801 fully inhibits formation of soluble amyloid oligomers = neurotoxic form of beta amyloid & avoids interaction with insoluble fibrillar plaque
- Robust brain penetration & target engagement using small molecule drives clinical benefits
- ALZ-801 enhances body’s natural defense against formation of toxic amyloid oligomers
- Oral tablet designed to slow or stop disease progression – addressed by ALZ-8012
- Inhibits brain shrinkage associated with disease onset & progression
- Favorable safety observed across clinical trials without brain edema caused by some antibodies
- Accurate patient selection by application of precision medicine – addressed by ALZ-8013
- Genetic testing identified patients at high risk for AD & highly responsive to our medication
- Enhances accuracy of diagnosis – almost third of clinically diagnosed patients had no brain amyloid
1 Alzheon publications: Hey (2018) CNS Drugs; Hey (2018) Clin Pharmacokinet; Kocis (2017) CNS Drugs
2 Alzheon publications: Tolar (2021) Int J Mol Sci; Tolar (2020) Alzheimers Res Ther; Hey (2018) Clin Pharmacokinet
3 Alzheon publications: Tolar (2019) Alzheimers Dement; Abushakra (2017) J PrevAlz Dis; Abushakra (2016) J PrevAlz Dis

ALZ-801 (valiltramiprosate) profile superior to plaque-clearing antibodies
Only oral anti-amyloid drug in Phase 3 development