In the News
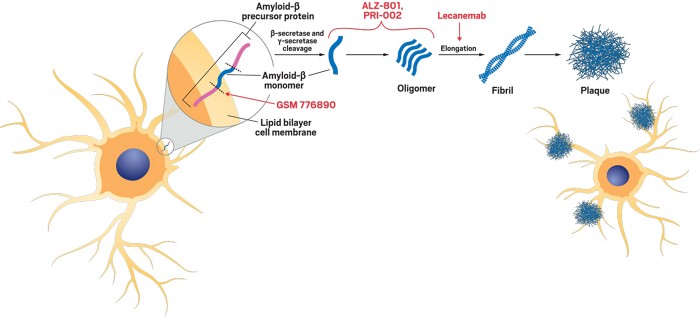
AAN 2023: ALZ-801 for Alzheimer’s found to improve cognition in trial
Alzheimer's News Today
The New Era of Life Sciences
Newsweek
Newsweek Investment Report: Interview with Dr. Martin Tolar, President & CEO of Alzheon, Inc.
Newsweek Investment Reports