In the News
Press Releases
Alzheon Raises $100 Million Series E Financing Round to Advance Development and Commercialization of Oral Tablet ALZ-801/Valiltramiprosate for Treatment of Alzheimer’s Disease
Proceeds Will Support Completion of Pivotal APOLLOE4 Phase 3 Study Evaluating ALZ-801/Valiltramiprosate and Regulatory Filings for Patients with Early Alzheimer’s Disease in 2024
Alzheon Announces Appointment of Renowned Biopharma Executive Gino Santini to Board of Directors
Mr. Santini’s Extensive Global Commercial and Business Development Experience Strengthens Alzheon’s Board Leadership
Alzheon Announces First Patient Dosed in Long-Term Extension of APOLLOE4 Phase 3 Trial of Oral ALZ-801/Valiltramiprosate and Launches 52-Week Extension of Phase 2 Biomarker Trial in Patients with Early Alzheimer’s Disease
APOLLOE4 Phase 3 Trial Subjects Who Completed 78 Weeks of Treatment Offered Enrollment in Long-Term Extension Study to Provide Insights into Effect of ALZ-801
Alzheon Announces that United States Federal Circuit Affirmed Decision of U.S. Patent Office Invalidating Risen (Suzhou) Pharmaceutical Technology Co., Ltd.’s Patent on Isotopically Enriched Forms of ALZ-801/Valiltramiprosate
U.S. Federal Circuit Affirmed Without Discussion Final Decision of U.S. Patent Office Invalidating Risen (Suzhou) Pharma Tech’s U.S. Patent No. 10,472,323 on Isotopically Enriched Forms of [...]
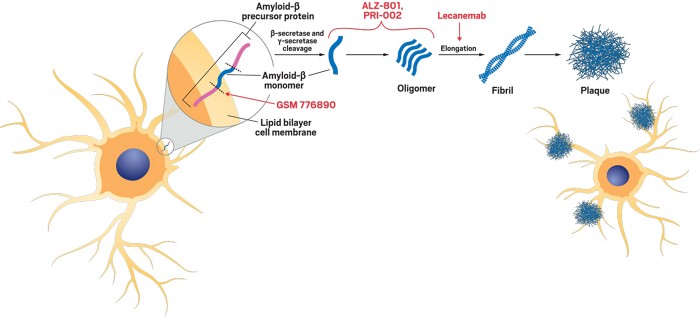
Peer-Reviewed Scientific Publication Proposes Unifying Single Toxin Theory of Brain Neurodegeneration that Identifies New Drug Targets and Treatments for Alzheimer’s Disease and Other Neurodegenerative Disorders
Soluble Beta Amyloid Aggregates Called Oligomers or Protofibrils Initiate and Drive Pathogenesis of Alzheimer’s Disease All Other Biochemical Effects and Neurodegenerative Changes Observed in Alzheimer’s Brain [...]
Alzheon to Present Biomarker, Brain Preservation, and Clinical Results from Pivotal Program of Oral ALZ-801/Valiltramiprosate at International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorders
ALZ-801 Tablet Inhibits Formation of Soluble Toxic Amyloid Aggregates and Acts Upstream from All Late-Stage Amyloid Targeting Treatments