Alzheon Pipeline
Our lead investigational product, valiltramiprosate/ALZ-801, completed APOLLOE4 Phase 3 study
Granted Fast Track Designation by FDA for development in Alzheimer’s disease
Focused on early symptomatic Alzheimer’s disease
Targeted patient selection based on APOE4/4 homozygote genotype
Ongoing long-term extension (LTE)
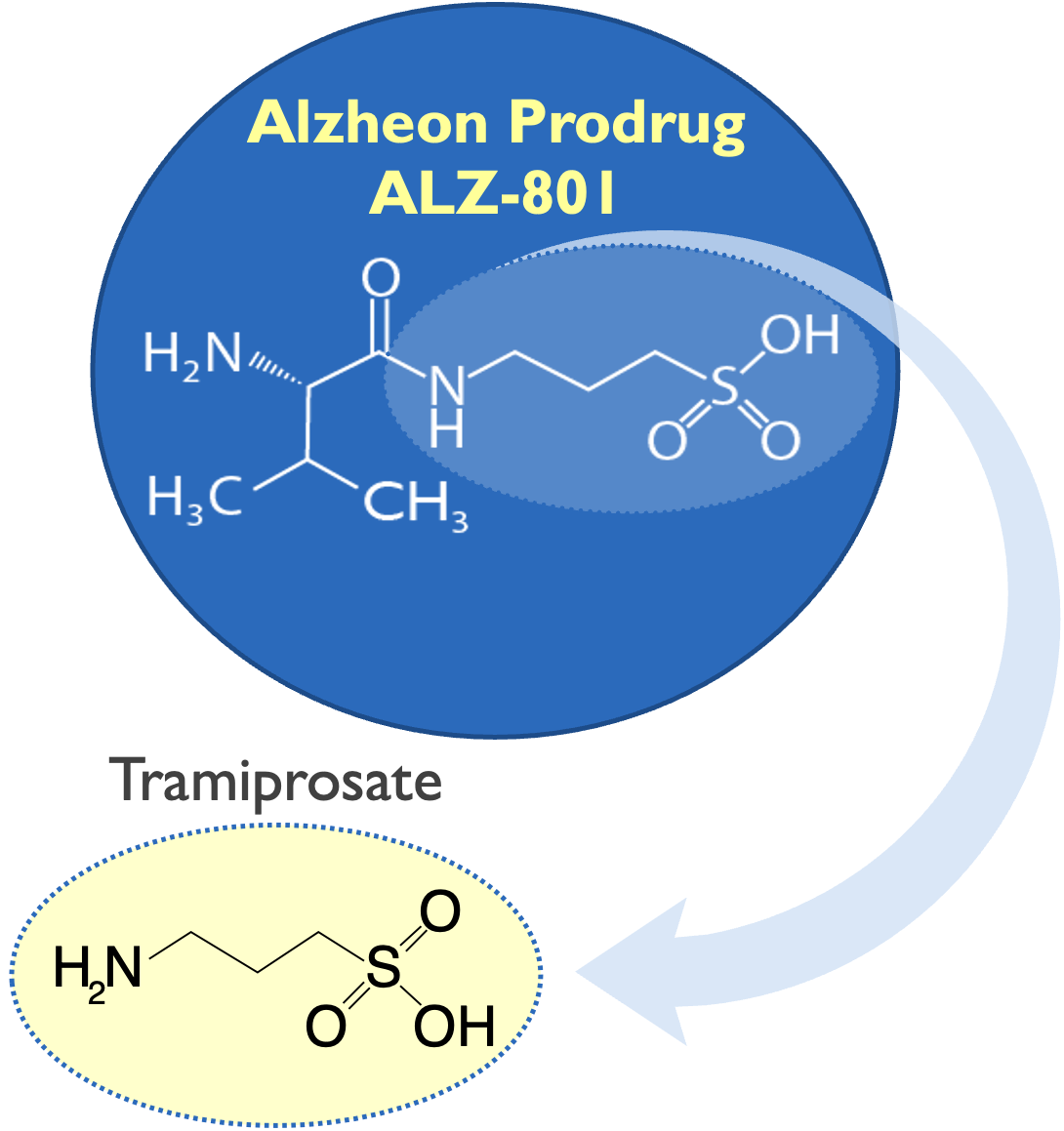
Valiltramiprosate is prodrug of tramiprosate
Promising clinical signals in a subset of Alzheimer’s patients
Long-term safety evaluated in ~2,000 AD patients
Discovery Platform
Compound libraries enabled by the novel mechanism of action
Designed to inhibit the misfolding of proteins associated with neurodegenerative diseases
Valiltramiprosate/ALZ-801 profile
Addition of valine substantially improved PK & tolerability in AD patients
- High, sustained brain levels ~40%*
- Lower inter-patient variability ~25%
- Improved GI tolerability vs. tramiprosate
- 265 mg tablet, twice daily fully inhibits Aβ oligomer formation
- Commercial-ready tablet in Phase 3 trial
*Brain to plasma ratio; GI – gastrointestinal