Applying precision medicine to find a better solution
About Alzheon
Alzheon is a clinical-stage biopharmaceutical company with a Phase 3 program in Alzheimer’s disease and a discovery platform of small molecules for the treatment of neurodegenerative disorders. We are developing these disease modifying treatments by leveraging our expertise in inhibiting protein misfolding and aggregation.
Alzheon company profile
- Founded in 2013 by Martin Tolar, MD, PhD
- Developing an investigational oral anti-amyloid oligomer treatment valiltramiprosate
- Completed Phase 2 biomarker open-label study supports disease modifying potential of valiltramiprosate
- Completed APOLLOE4 Phase 3 study evaluating valiltramiprosate oral tablet
- Received $51M in grants from National Institute on Aging to partially fund APOLLOE4 Phase 3 trial
Alzheon’s valiltramiprosate/ALZ-801 is a late-stage, investigational oral anti-amyloid treatment having just completed Phase 3 testing for early symptomatic Alzheimer’s disease.
Development of valiltramiprosate, is based on ten years of Alzheon research into the biology of Alzheimer’s.
Using our pioneering precision medicine approach, we are initially focusing our development on the highest risk patients with two copies of the APOE4 gene (APOE4/4 homozygotes). Future trials aim to expand treatment to patients with only one copy of the APOE4 gene.
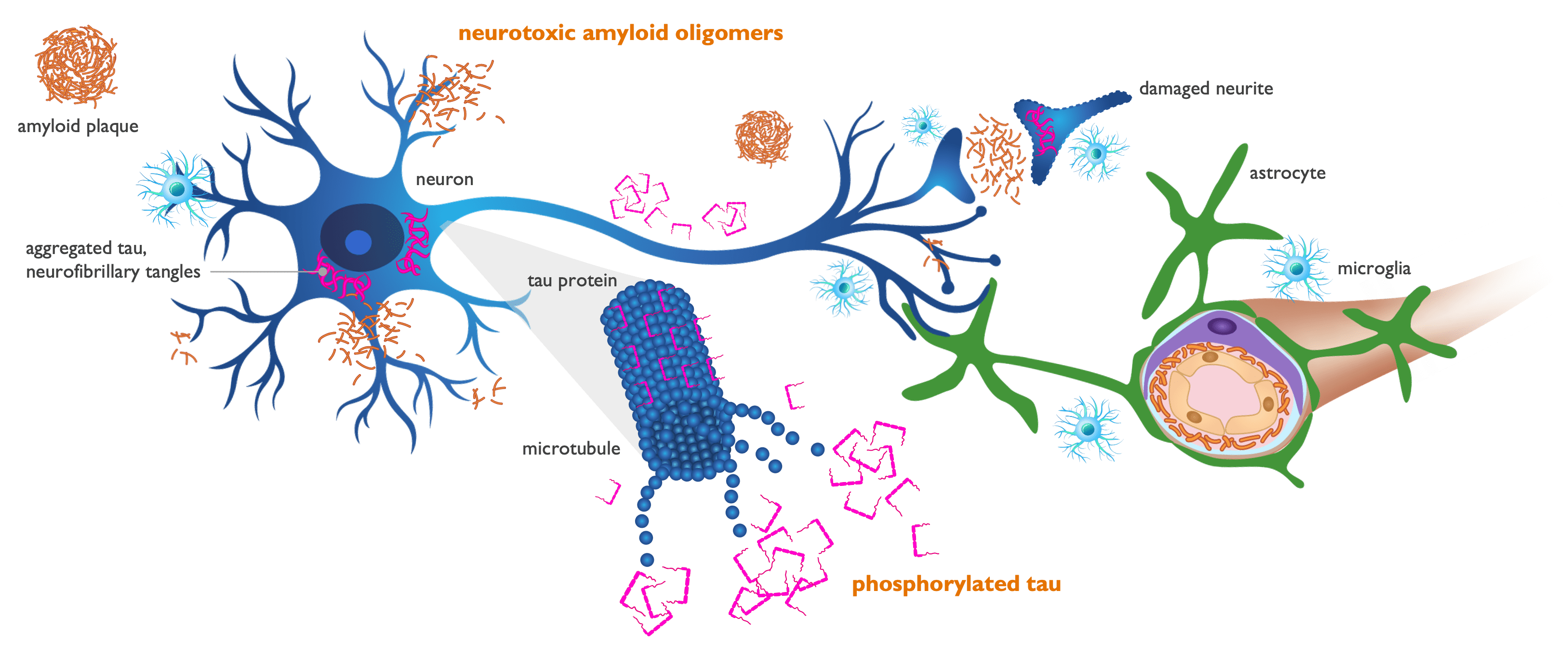
Amyloid oligomers drive neurotoxicity & tau pathology
Injured neurons release misfolded tau protein that aggregates into tangles