Press Releases
News Coverage
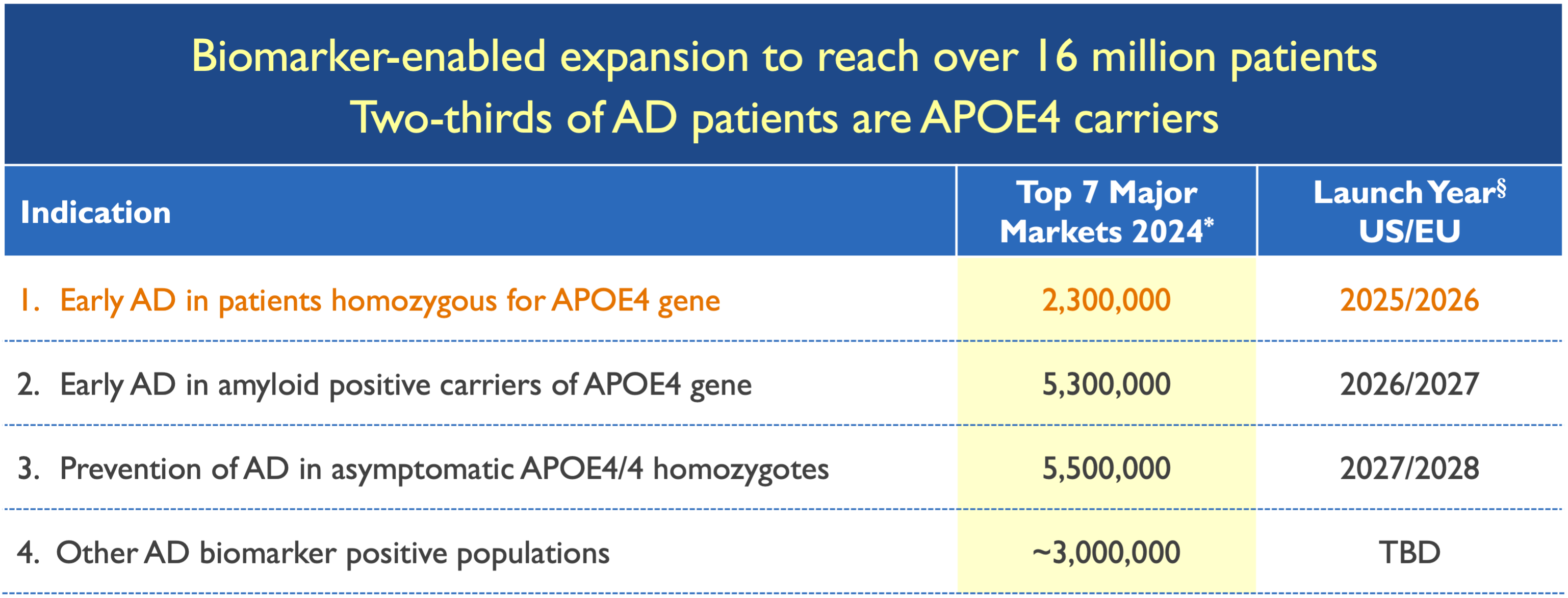
6.2M people suffer from Alzheimer’s in the U.S. alone1
35 million Alzheimer’s patients worldwide2
1 Alzheimer’s Association 2021
2 World Alzheimer’s Reports 2020
3 Wall Street research
4 Current market only symptomatic medications ~$6.5B in 2015
*Only 4 DM programs in late-stage development
ALZ-801 could be the first oral disease modifying treatment for Alzheimer’s
- Alzheimer’s disease = 6th leading cause of death
- A growing problem in U.S. due to aging population
- Disease characterized by
- Destructive memory loss, impaired ability to communicate & comprehend
- Inability to complete simple, familiar tasks, confusion with time & place
- Poor judgment & decision-making, becoming suspicious, depressed or anxious
- Death within 5 to 8 years from emergence of clinical symptoms
- Disease characterized by
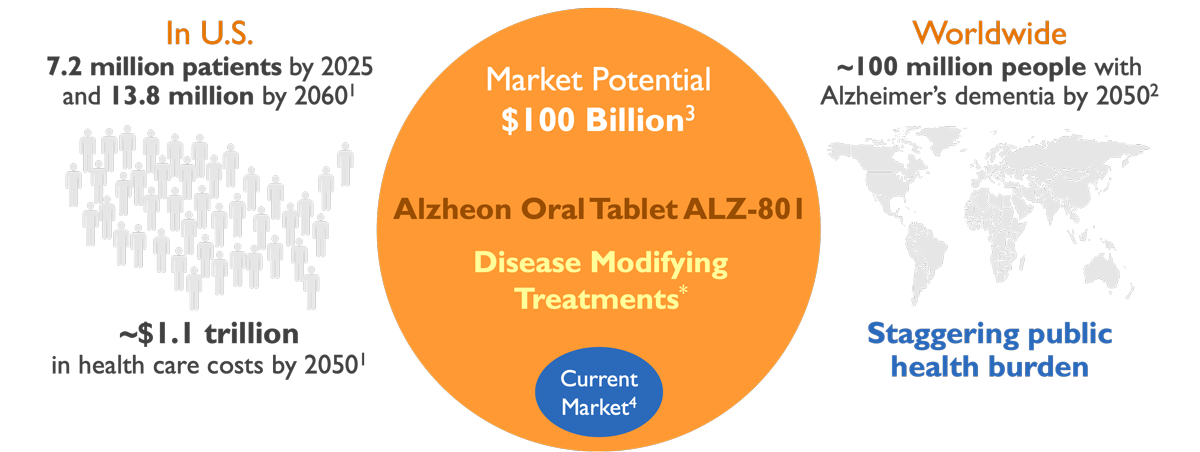
- Currently 5.8 million people in U.S. suffer from Alzheimer’s disease
- Number of patients will increase by ~70% over the next 12 years
- 47 million people with initial changes in the brain but not yet symptomatic
- Direct healthcare costs = $277 billion (mostly Medicare)
- Family & caregiver burden: 16 million people provide unpaid care at cost of $232 billion
Alzheimer’s Association 2018; Brookmeyer 2017; World Alzheimer’s Reports 2018 & 2016
Source: Decision Resources Group: Disease Landscape & Forecast. Alzheimer’s Disease, Market Outlook, 2019
Unprecedented Commercial Opportunity for Alzheimer’s Disease Modifying Treatments
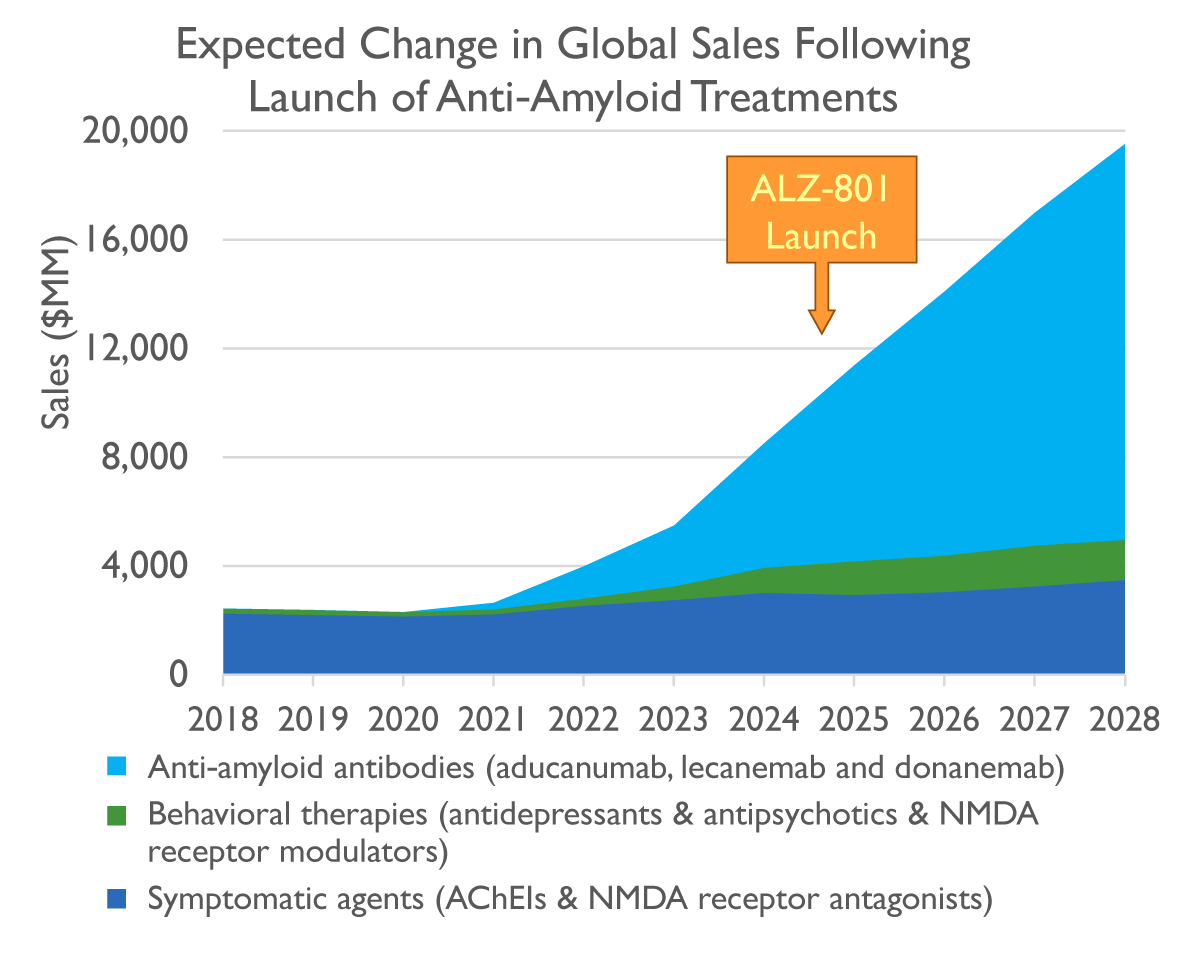
Disease-modifying therapies forecast to drive growth: $15B+ in sales by 2028
- Surge in early AD diagnosis & treatment
- Aducanumab (Aduhelm, Biogen): modest efficacy with safety limitations
- Lecanemab (Eisai), donanemab (Eli Lilly), gantenerumab (Roche) Phase 3 ongoing
Limited uptake of antibodies
- Modest efficacy
- Safety risks of brain edema & hemorrhage
- Invasive, burdensome injections & infusions
- Cost & access restrictions
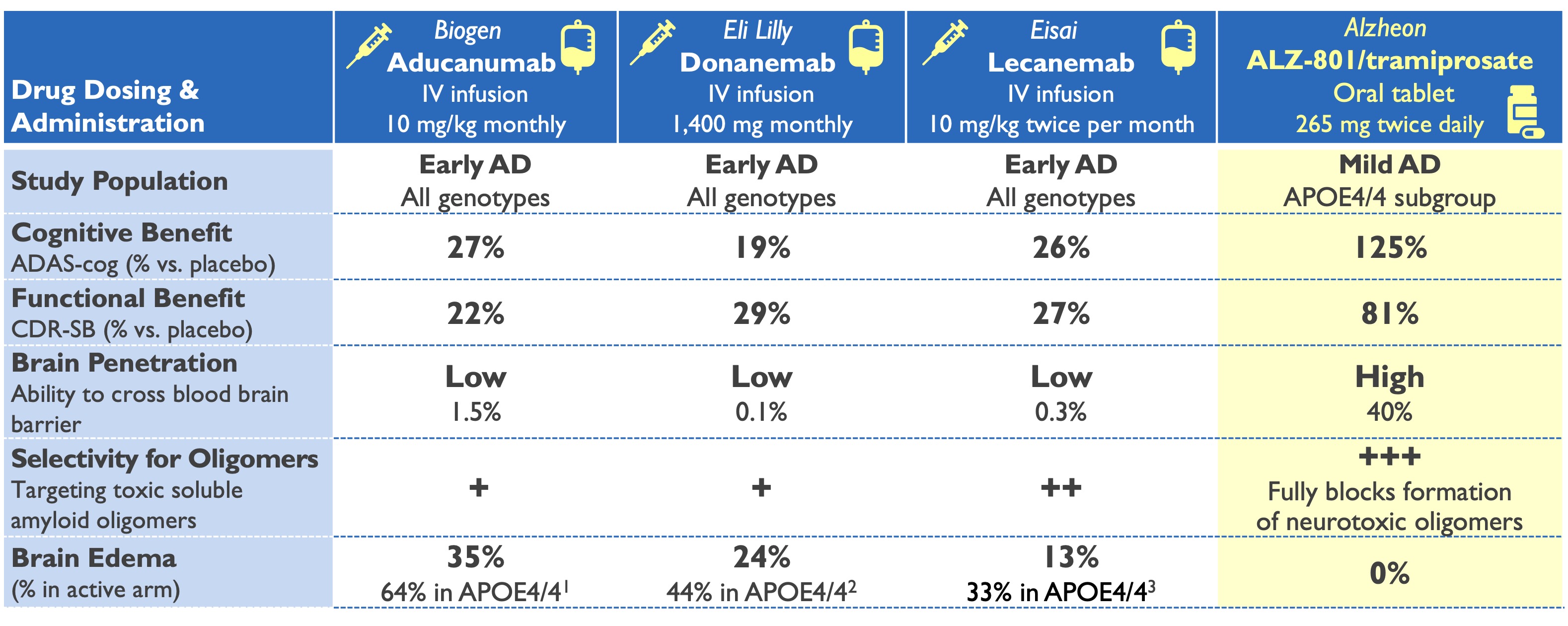
ALZ-801 product profile
- Robust efficacy in cognition & daily function*
- Disease stabilized for 18-month trial duration
- Preserved hippocampal volume
- Persistent clinical benefits over 2.5 years
- Well-tolerated oral tablet ideally suited for AD patients & their families
- No brain edema
- Low incidence of mild transient nausea
- No burdensome IV infusions or need for MRI monitoring for brain edema
- Aducanumab approval validates targeting toxic amyloid**
- Safety data highlight need for APOE4 testing linked to risk of ARIA
- No other requirement for diagnostic testing: no PET scans or CSF biomarkers
*Based on tramiprosate Phase 3 data in Mild AD APOE4/4 patients, may be improved based on superior profile of ALZ-801 vs. tramiprosate
**Aduhelm Prescribing Information, U.S. FDA 2021 & Aducanumab Biologics License Application: Medical Safety Review, June 2021

Alzheon publications: Tolar (2021) Int J Mol Sci; Tolar (2020) Alzheimers Res Ther; Tolar (2019) Alzheimers Dement
1Aducanumab Biologics License Application: Medical Safety Review, June 2021; 2Van Dyck NEJM 2023 & Lecanemab Ph3 presentation ADPD 2023
3Eli Lilly: Donanemab Phase 3 Press Release, May 2023 & Phase 2 study Mintun NEJM 2021
ALZ-801 (valiltramiprosate) profile superior to plaque-clearing antibodies
Only oral anti-amyloid drug in Phase 3 development
Exceptional Revenue Potential of First Oral AD Treatment