Applying precision medicine to find a better solution
Alzheon’s ALZ-801 (valiltramiprosate) is currently the only oral anti-amyloid treatment in Phase 3 testing for Alzheimer’s disease. We believe that ALZ-801 has the potential to significantly slow or stop disease progression, and even prevent the onset of clinical symptoms.
Development of ALZ-801, a well-tolerated new treatment, is based on ten years of Alzheon research into the biology of Alzheimer’s. Safety profile in ALZ-801 studies remains favorable & consistent with prior data in over 3,000 AD patients, with no evidence of vasogenic brain edema.
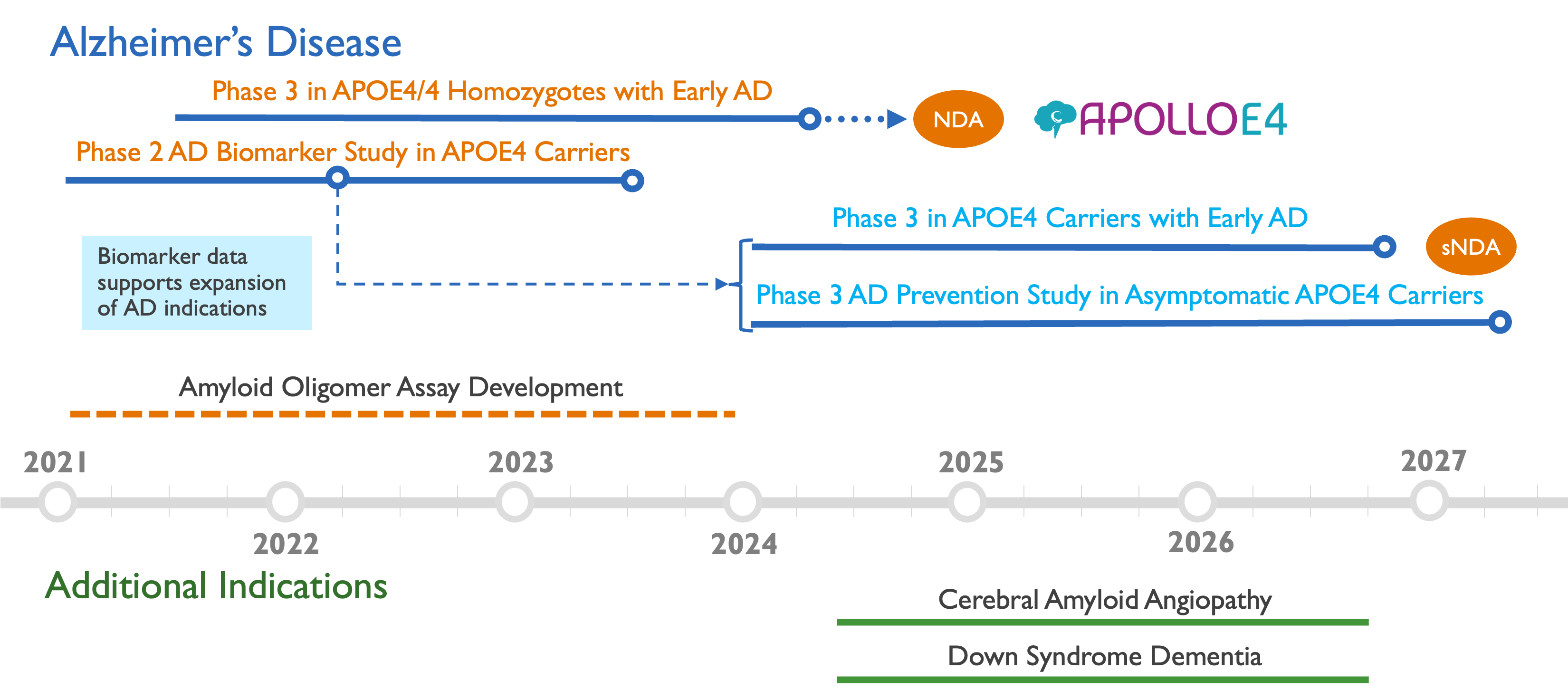
Using our pioneering precision medicine approach, we are initially focusing our development on the highest risk patients with two copies of the APOE4 gene (APOE4/4 homozygotes), who are predicted to benefit most from the ALZ-801 treatment. Future trials will expand treatment to patients with only one copy of the APOE4 gene, and ultimately to prevention of onset of Alzheimer’s.
Wall Street analyst Robyn Karnauskas of Truist Securities discussing with Alzheon management team new data and therapeutic potential of ALZ-801/valiltramiprosate.
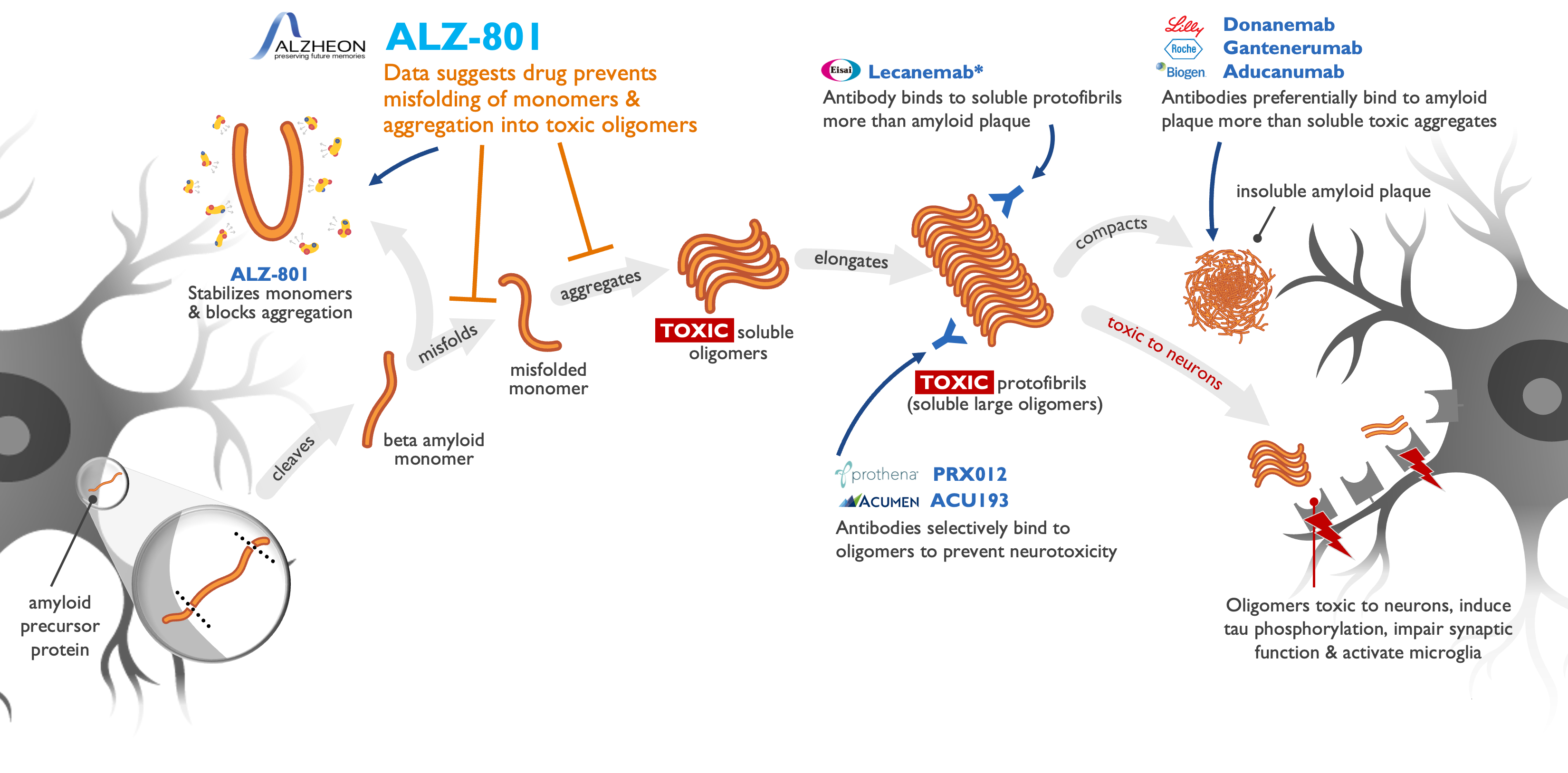
ALZ-801 acts upstream of anti-amyloid drugs in AD
Validated approach supported by experts

- Received $51M in grants from National Institute on Aging to fund APOLLOE4 Phase 3 study
- Prominent global Scientific Advisory Board
- Alzheon published 9 peer reviewed scientific publications on key discoveries in Alzheimer’s
Phase 2 trial Biomarker Steering Committee: the top 3 experts
| Kaj Blennow, MD, PhD Developed p-tau181 biomarker for AD (on Aduhelm’s FDA label); Professor of Clinical Neurochemistry, University of Gothenburg, Sweden |
Eric Reiman, MD Developed p-tau217 biomarker for AD; Executive Director of Banner Alzheimer’s Institute, Chief Executive Officer of Banner Research, Phoenix |
Philip Scheltens, MD, PhD Developed imaging & fluid AD biomarkers; Managing Partner of LSP Dementia Fund; Director of the Alzheimer Center, VU University, Amsterdam, Netherlands |
|---|
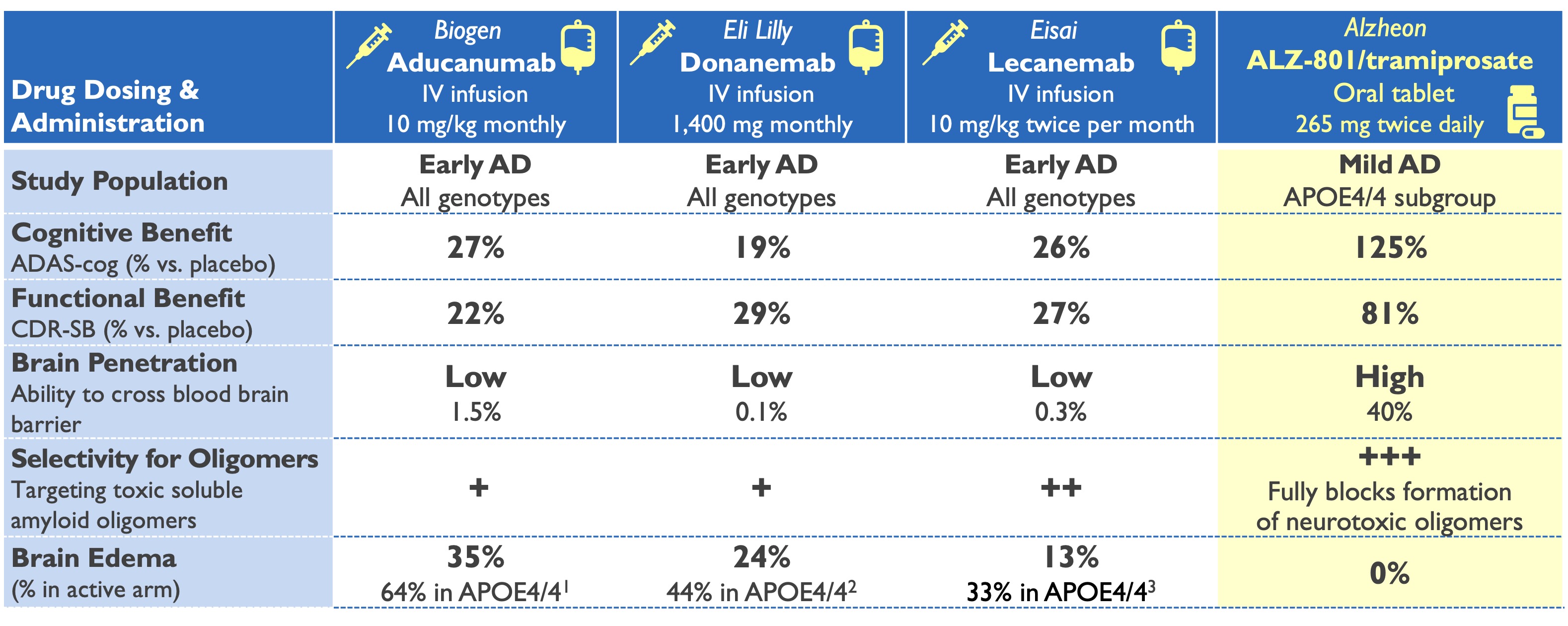
ALZ-801 (valiltramiprosate) profile superior to plaque-clearing antibodies
Only oral anti-amyloid drug in Phase 3 development
Alzheon publications: Tolar (2021) Int J Mol Sci; Tolar (2020) Alzheimers Res Ther; Tolar (2019) Alzheimers Dement
1Aducanumab Biologics License Application: Medical Safety Review, June 2021; 2Van Dyck NEJM 2023 & Lecanemab Ph3 presentation ADPD 2023
3Eli Lilly: Donanemab Phase 3 Press Release, May 2023 & Phase 2 study Mintun NEJM 2021
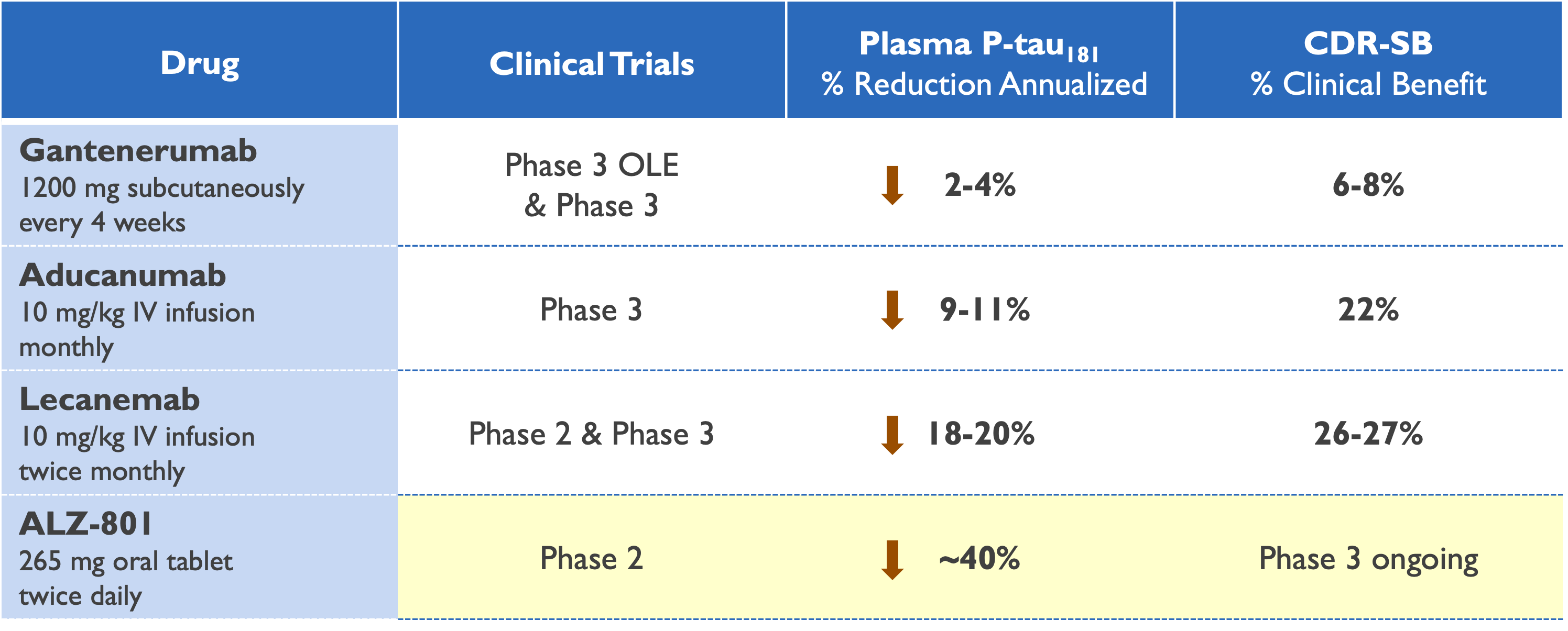
Gantenerumab – AAIC 2022; Roche GRADUATE Phase 3 Studies Press Release, November 2022
Aducanumab – AAIC 2021 & CTAD 2021
Lecanemab – CTAD 2021; Swanson (2020) Alz Res Therapy; Eisai CLARITY AD Phase 3 Study Press Release, September 2022
ALZ-801 – CTAD 2022
Plasma p-tau181 reduction predicts clinical efficacy
ALZ-801 (valiltramiprosate) development strategy